Carrier Screening Market Forecast Report 2034: Key Segments, Regional Expansion, and Industry Leaders
Key companies covered in the Carrier Screening Market are Natera, Inc., Labcorp, Quest Diagnostics Incorporated, Myriad Genetics, Inc., and others.
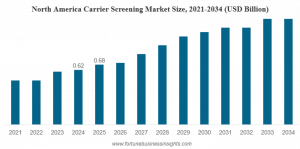
The global carrier screening market size was valued at USD 1.70 billion in 2025 and is projected to grow from USD 1.88 billion in 2026 to USD 3.08 billion by 2034.”
PUNE, MAHARASHTRA, INDIA, February 10, 2026 /EINPresswire.com/ -- The Carrier Screening Market Size reflects a rapidly expanding healthcare segment driven by growing genetic testing needs, rising awareness of inherited conditions, and advances in sequencing technologies. According to the latest report from Fortune Business Insights, the global carrier screening market was valued at USD 1.70 billion in 2025 and is expected to grow from USD 1.88 billion in 2026 to USD 3.08 billion by 2034, exhibiting a Compound Annual Growth Rate (CAGR) of 6.4% during the forecast period. — Fortune Business Insights
Carrier screening involves genetic tests that identify whether an individual carries a gene for a specific inherited disorder, even if they do not exhibit symptoms themselves. These tests are particularly essential for couples planning pregnancy or during prenatal care, as they help assess the risk of passing on recessive genetic disorders to offspring. The rising prevalence of genetic diseases, coupled with increasing use of advanced technologies such as next-generation sequencing (NGS), is expanding the adoption of carrier screening tests worldwide.
Market Growth Factors
Several key Market Growth Factors are accelerating the global expansion of the carrier screening market. One major driver is the increasing prevalence of genetic disorders across populations, which has heightened demand for early detection and preventive healthcare. For instance, data from health research organizations indicate millions of births each year are affected by genetic defects, underscoring the need for carrier screening to inform reproductive decisions and reduce health risks.
Technological advancements also play a critical role in shaping market growth. The widespread adoption of next-generation sequencing (NGS) technologies has made carrier screening more comprehensive and cost-effective, enabling simultaneous analysis of large gene panels that detect hundreds of conditions regardless of ethnic or family history. This expanded carrier screening (ECS) approach is gaining prominence due to its thorough detection capabilities, which improve clinical outcomes and patient counseling.
Get a Free Sample of this Report:
https://www.fortunebusinessinsights.com/enquiry/request-sample-pdf/carrier-screening-market-114944
Top Companies in Market:
• Natera, Inc. (U.S.)
• Labcorp (U.S.)
• Quest Diagnostics Incorporated. (U.S.)
• Myriad Genetics, Inc. (U.S.)
• Fulgent Genetics (U.S.)
• BGI Group (China)
• Berry Genomics (China)
• Eurofins Scientific (Luxembourg)
• CENTOGENE GmbH (Germany)
• YOURGENE HEALTH (U.K.)
• MedGenome (India)
Market Trends
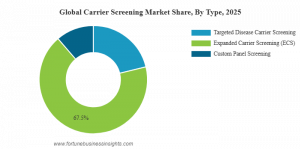
The Market Trends in carrier screening show a shift toward broader panel testing and personalized reproductive healthcare. Expanded carrier screening, which enables testing across an extensive range of inherited conditions in a single test, is emerging as the dominant segment due to its pan-ethnic utility and preventive value. Many clinics and testing providers now recommend ECS panels as a standard preconception assessment, which is also reflected in growing clinical guidelines endorsement.
Further, the integration of NGS and related high-throughput technologies continues to reduce per-test costs while improving accuracy and turnaround times. As sequencing platforms become more accessible and affordable, more laboratories are adopting advanced genetic analysis solutions, which expands service offerings and increases patient access to carrier screening.
Market Segmentation Analysis
By Type: The carrier screening market includes expanded carrier screening (ECS), targeted disease carrier screening, and custom panel screening. Among these, expanded carrier screening is expected to dominate the market due to its ability to assess a wide range of genetic conditions irrespective of ethnicity or family history, increasing its clinical utility and adoption.
By Technology: Key technological segments include next-generation sequencing (NGS), polymerase chain reaction (PCR), microarray-based screening, and other molecular diagnostics techniques. NGS holds the largest share and is rapidly driving innovation due to its broader detection capabilities and falling sequencing costs, making comprehensive carrier screening more feasible for both providers and patients.
By Indication: Carrier screening tests are used to detect carriers of various inherited conditions such as hemoglobinopathies, cystic fibrosis, spinal muscular atrophy (SMA), fragile X syndrome, and other genetic diseases. The high prevalence of hemoglobinopathies in global populations contributes significantly to market demand.
By Sample Type: The market is segmented into blood-based screening, saliva-based testing, and other sample types, reflecting diverse clinical workflows and patient preferences. Blood remains a widely used sample type due to its reliability and established protocols in clinical practice.
By Service Provider: Service delivery channels include clinical laboratories, hospital laboratories, direct-to-consumer (DTC) testing companies, and others. Clinical laboratories lead in service volumes due to their extensive infrastructure and integration with healthcare systems.
Ask for Customization:
https://www.fortunebusinessinsights.com/enquiry/customization/carrier-screening-market-114944
Regional Insights
REGIONAL INSIGHTS show North America commanding a dominant share of the global carrier screening market. In 2025, North America’s market value was approximately USD 0.68 billion, reflecting strong adoption of genetic testing, robust healthcare infrastructure, favorable reimbursement policies, and high public awareness of genetic disorders. The United States, specifically, continues to lead regional growth with increasing test volumes and expanded clinical guidelines promoting carrier screening.
Europe is also poised for steady growth, driven by increased adoption of ECS panels and broader genetic testing infrastructure. Countries like Germany, France, and the U.K. are witnessing rising carrier screening utilizations as part of prenatal and reproductive healthcare services.
The Asia Pacific region is expected to emerge as a significant growth market as healthcare access expands and public awareness of genetic conditions increases. China, India, and other emerging economies are investing in genetic testing services and prenatal care, supporting broader adoption of carrier screening. Latin America and the Middle East & Africa are projected to show incremental growth as healthcare modernization, and prenatal care initiatives improve genetic testing penetration over time.
Key Industry Developments
• October 2024 – Yourgene Health (part of the Novacyt group of companies), a leading international molecular diagnostics group, announces that it has received accreditation under the new EU requirements of the in vitro diagnostic regulation (IVDR) for the Yourgene Cystic Fibrosis Base assay.
• January 2024 – Natera, Inc., a global leader in cell-free DNA (cfDNA) testing, announced that it has acquired reproductive health assets from Invitae. These assets relate to Invitae’s non-invasive prenatal screening and carrier screening business.
Read Related Reports:
Companion Diagnostics Market Size, Share
Digital Pathology Market Size, Share, Growth
Ashwin Arora
Fortune Business Insights™ Pvt. Ltd.
+1 833-909-2966
sales@fortunebusinessinsights.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.